FDA approves new Alzheimer's treatment, donanemab from Eli Lilly
- EP News Service
- Jul 05, 2024
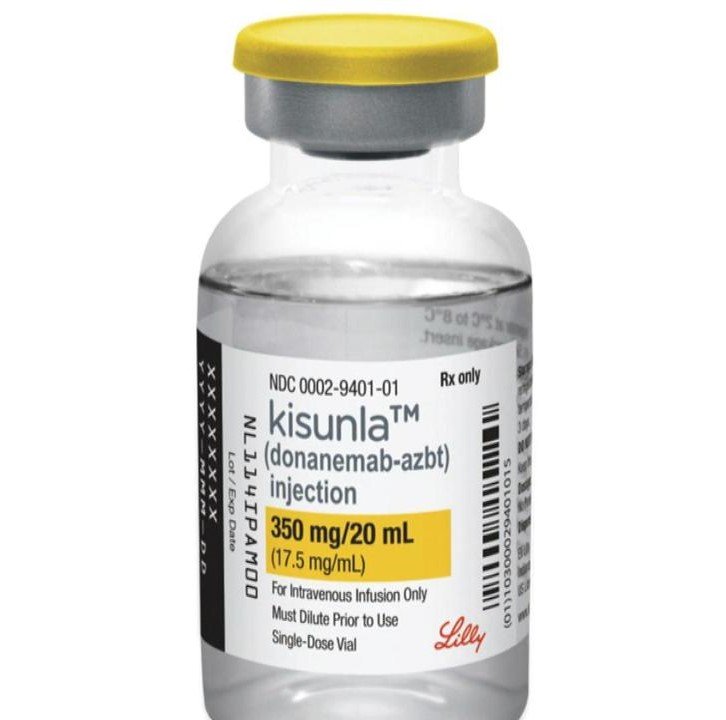
Branded as Kisunla by drugmaker Eli Lilly, donanemab gets FDA go ahead
NEW DELHI: The U.S Food and Drug Administration (FDA) has approved a new Alzheimer's treatment called donanemab, clearing the way for the third addition to a new class of drugs aimed at slowing the brain's decline in patients facing the early stages of the disease.
Branded as Kisunla by drugmaker Eli Lilly, donanemab's approval follows years of setbacks and delays in getting the experimental Alzheimer's treatment to market, despite promising clinical trial results. Eli Lilly says the drug will be available within weeks following the approval.
"Kisunla demonstrated very meaningful results for people with early symptomatic Alzheimer's disease, who urgently need effective treatment options. We know these medicines have the greatest potential benefit when people are treated earlier in their disease, and we are working hard in partnership with others to improve detection and diagnosis," Anne White, president of Eli Lilly's neuroscience arm, said in a news release.
The FDA previously rebuffed Eli Lilly's request for accelerated approval last year, citing concerns about its long-term safety data. After Eli Lilly submitted more data to the FDA, the company said it expected the agency would decide on approval by the end of March.
That decision was delayed after the FDA scheduled an advisory committee to wrestle with questions over the drug's safety issues and how effectiveness was measured in its trials. The panel ultimately voted unanimously last month in favour of the drug's benefits outweighing its risks, for patients in the early stages of Alzheimer's disease.










Reporter
Crisp, and to the point news coverage from India and around the world.
View Reporter News